3 min read
As stated in our blog about Mature Quality Management Systems, quality is the foundation of a successful business. In this blog we cover the important differences between corrective action and preventive action, the purpose of the CAPA subsystem and FDAs expectations from your CAPA process.
Let us start with the basics: what is CAPA?
CAPA is the abbreviation for corrective action and preventive action. CAPA was introduced as a component of the Quality Systems Guidance. This guidance forms the basis of the ICH Guideline Q10, laying out the CAPA process within the pharmaceutical space.
For medical device companies, CAPA is addressed in ISO 13485, which unlike Q10, divides the concept into its two concepts: “Corrective measures” and “Preventive measures”.
What is the difference between Corrective Action and Preventive Action?
- A corrective action is a reaction to a problem that has already occurred. An action to eliminate the cause of a detected non-conformity or other undesirable situation. Corrective is reactive and reported by either internal or external sources.
- A preventive action is initiated to eliminate the cause of a potential non-conformity or other undesirable situation. Preventive is proactive, it assumes that adequate monitoring and controls are in place in the quality system to assure that potential problems are identified and eliminated before they happen.
What is the purpose of the CAPA subsystem?
- collect information
- analyze information
- identify and investigate product and quality problems
- take appropriate and effective corrective and / or preventive action to prevent their recurrence
- verifying or validating corrective and preventive actions
- communicating corrective and preventive action activities to responsible people
- providing relevant information for management review
- documenting these activities
What FDA expects from your CAPA process:
“Manufacturers should consider that their Corrective Action and Preventive Action documentation can demonstrate to FDA that the manufacturer’s quality system is effective and enables the manufacturer to identify problems quickly and implement effective corrective and preventive actions.”
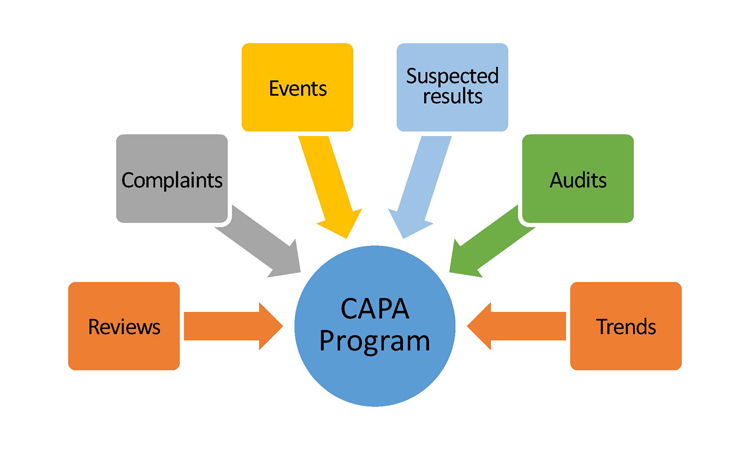
An effective and compliant CAPA system requires a strong set of data in order to detect problems, find and implement solutions, and document findings and subsequent changes. Examples of data sources may include;
- Internal data sources
- process control data
- test/inspection data
- device history records
- internal audits
- nonconforming material reports
- rework and Scrap/Yield data
- training records
- External data sources
- supplier controls
- customers
- complaints
- servicing repairs
- adverse event reporting
- FDA
- similar devices from competitors
10 checkmarks – when evaluating your CAPA processes for compliance with FDA regulations.
- Verify that CAPA system procedure(s) that address the requirements of the quality system regulation have been defined and documented.
- Determine if appropriate sources of product and quality problems have been identified. Confirm that data from these sources are analyzed to identify existing product and quality problems that may require corrective action.
- Determine if sources of product and quality information that may show unfavorable trends have been identified. Confirm that data from these sources are analyzed to identify potential product and quality problems that may require preventive action.
- Challenge the quality data information system. Verify that the data received by the CAPA system are complete, accurate and timely.
- Verify that appropriate statistical methods are employed (where necessary) to detect recurring quality problems. Determine if results of analyses are compared across different data sources to identify and develop the extent of product and quality problems.
- Determine if failure investigation procedures are followed. Determine if the degree to which a quality problem or nonconforming product is investigated is commensurate with the significance and risk of the nonconformity. Determine if failure investigations are conducted to determine root cause (where possible). Verify that there is control for preventing distribution of nonconforming product.
- Determine if appropriate actions have been taken for significant product and quality problems identified from data sources.
- Determine if corrective and preventive actions were effective and verified or validated prior to implementation. Confirm that corrective and preventive actions do not adversely affect the finished device.
- Verify that corrective and preventive actions for product and quality problems were implemented and documented.
- Determine if information regarding nonconforming product and quality problems and corrective and preventive actions has been properly disseminated, including dissemination for management review.
In conclusion, step one toward building a CAPA subsystem that is compliant, effective and efficient is ensuring that your procedures are aligned with the expectations of regulators.
To find out more about how Pinnaql can help move you along your quality journey, contact us today.
Sources
https://www.fda.gov/corrective-and-preventive-actions-capa#page2
http://asq.org/quality-progress/2005/03/problem-solving/corrective-vs-preventive-action.html
https://ispe.org/pharmaceutical-engineering/september-october-2018/capa-maturity
